
Chemistry, 20.06.2020 08:57 graciewyatt471
1) How many kJ are absorbed when 45.2 g of water at 31.3 oC is heated to 76.9 oC? 2) Calculate the total heat released in kcal when 72.1 g water at 25.2 oC is cooled to 0 oC and freezes. 3) How many kilojoules are required to heat 55,500 mg of gold with specific heat = 0.129 J/g oC is heated from 24.6 oC to 123.4 oC? 4) Calculate the heat needed in kcal to change 45.6 g of water at 100 oC to change into steam.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1



Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
1) How many kJ are absorbed when 45.2 g of water at 31.3 oC is heated to 76.9 oC? 2) Calculate the t...
Questions in other subjects:









English, 19.08.2020 01:01

History, 19.08.2020 01:01

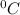 and Δθ is the change in temperature.
and Δθ is the change in temperature.

