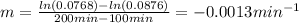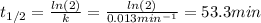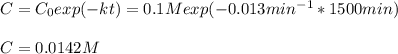3.The following data of decomposition reaction of thionyl chloride (SO2Cl2) were collected at a certain temperature and the concentration of SO2Cl2 were monitored as shown in the table.
SO2Cl2 (g) SO2 (g) + Cl2 (g)
Time (min)Conc. of SO2Cl2 (mol/L)
00.1000
1000.0876
2000.0768
3000.0673
4000.0590
5000.0517
6000.0453
7000.0397
8000.0348
9000.0305
10000.0267
11000.0234
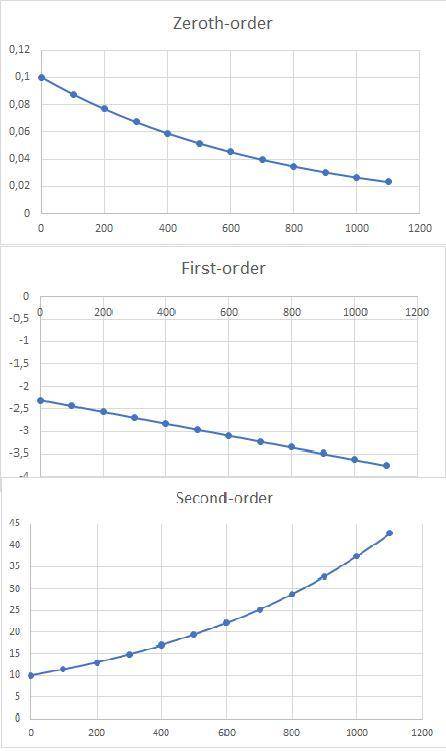
a)Determine graphically whether the kinetics of the reaction is zero order, first order or second order with respect to SO2Cl2 and then write the rate equation.
b)Determine the rate constant (k) of the reaction.
c)Determine the half-life (t½) for the reaction.
d)What will be the concentration of SO2Cl2 left in the reaction mixture at 1500 minutes?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, smartie80
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
3.The following data of decomposition reaction of thionyl chloride (SO2Cl2) were collected at a cert...
Questions in other subjects:



Mathematics, 18.11.2020 17:30


Business, 18.11.2020 17:30





Mathematics, 18.11.2020 17:30