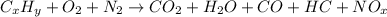Chemistry, 19.06.2020 05:57 UltimateGoal
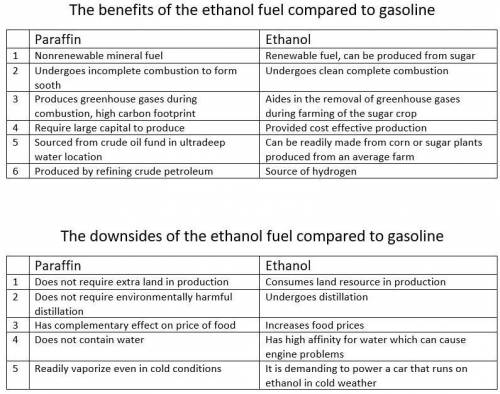
2. Paraffin and gasoline are both hydrocarbons, and therefore have the same general
chemical formula CnHn+2. As such, paraffin and gasoline are similar in that both do
not contain oxygen. Compare the use of gasoline (which would be similar to
paraffin) versus ethanol as a fuel, considering that gasoline is a hydrocarbon
whereas ethanol also contains oxygen. Compare the use of gasoline versus ethanol
as a fuel, from the standpoint of efficiency, by comparing the amount of energy that
each contains on a per amount of fuel basis.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
2. Paraffin and gasoline are both hydrocarbons, and therefore have the same general
chemical form...
Questions in other subjects:


Mathematics, 08.02.2021 23:10



History, 08.02.2021 23:10

Mathematics, 08.02.2021 23:10



Biology, 08.02.2021 23:10

Mathematics, 08.02.2021 23:10