Chemistry, 19.06.2020 00:57 josephvcarter
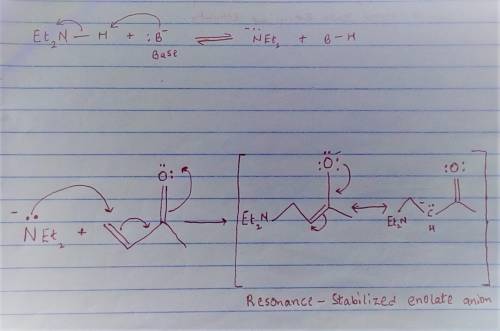
Carbon-carbon double bonds are electron-rich regions and are attacked by electrophiles (for example, ); they are not attacked by nucleophiles (for example, diethylamine, ). + reaction arrow with electrophilic addition written above + no reaction However, when the carbon-carbon double bond has a carbonyl group adjacent to it, the double bond reacts readily with nucleophiles by nucleophilic addition. + reaction arrow with nucleophilic addition written above For the following reaction, draw the structure of the resonance contributor that is attacked by diethylamine. + Include all valence lone pairs in your answer.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Carbon-carbon double bonds are electron-rich regions and are attacked by electrophiles (for example,...
Questions in other subjects:


History, 31.08.2021 04:10

Mathematics, 31.08.2021 04:10

Mathematics, 31.08.2021 04:10



Computers and Technology, 31.08.2021 04:20

Mathematics, 31.08.2021 04:20


History, 31.08.2021 04:20

 - carbon of the conjugate thereby resulting into a resonance stabilized enolate anion.
- carbon of the conjugate thereby resulting into a resonance stabilized enolate anion.