
A 1.8 g mass of fructose is added to 0.100 kg of water and it is
found that the freezing point has decreased by 0.186 °C. Given
that the Kf value of water is 1.86 °C kg/mol, what is the molar
mass of fructose (van't Hoff factor, i = 1)?
g/mol
Round your answer to the nearest whole number. Do not include units in your answer

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 09:00, hellodarkness14
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
You know the right answer?
A 1.8 g mass of fructose is added to 0.100 kg of water and it is
found that the freezing point has...
Questions in other subjects:




English, 28.07.2019 12:00


Mathematics, 28.07.2019 12:00




Mathematics, 28.07.2019 12:00

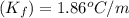

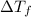 = change in freezing point =
= change in freezing point = 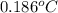
 = freezing point constant =
= freezing point constant = 



