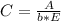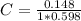Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
After creating a Beer's Law plot using standard solutions of Q, you determined the slope of Beer's L...
Questions in other subjects:

Mathematics, 12.03.2021 19:40






Mathematics, 12.03.2021 19:40


English, 12.03.2021 19:40

History, 12.03.2021 19:40

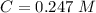
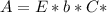 . On this equation we will have:
. On this equation we will have: