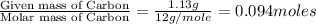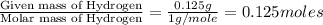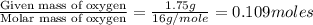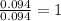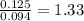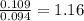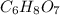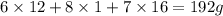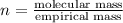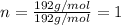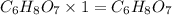A 3.00g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 192./gmol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 4.13g water 1.13g Use this information to find the molecular formula of X.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 23.06.2019 01:30, kenldykido2300
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 04:00, izzyp619
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
A 3.00g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to hav...
Questions in other subjects:


Biology, 08.07.2019 02:30



Mathematics, 08.07.2019 02:30

History, 08.07.2019 02:30




Mathematics, 08.07.2019 02:30

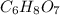
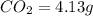
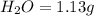
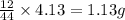 of carbon will be contained.
of carbon will be contained.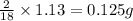 of hydrogen will be contained.
of hydrogen will be contained.