
Chemistry, 16.06.2020 10:57 simoneee89
Determine the hydroxide ion concentration in a solution that is: 1 × 10-4 M HCl. (kw = 1.0 X10-14)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
Determine the hydroxide ion concentration in a solution that is: 1 × 10-4 M HCl. (kw = 1.0 X10-14)...
Questions in other subjects:

History, 13.10.2020 05:01


Mathematics, 13.10.2020 05:01






Mathematics, 13.10.2020 05:01


![pH=-\log [H^+]](/tpl/images/0686/8556/37e81.png)
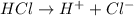
 gives = 1 mole of
gives = 1 mole of 
 moles of
moles of  moles of
moles of ![K_w=[H^+][OH^-]](/tpl/images/0686/8556/bc68a.png)
![10^{-14}=[1\times 10^{-4}][OH^-]](/tpl/images/0686/8556/a69e8.png)
![[OH^-]=1\times 10^{10}M](/tpl/images/0686/8556/5d6e6.png)



