
Chemistry, 17.06.2020 05:57 maciemessing2
The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments. The amino group of glycine, which has a pKa of 9.6, can exist either in the protonated form (-NH3+) or as the free base (−NH2), because of the reversible equilibrium:
R-NH3+ left right double arrow R−NH2 + H+
A) In what pH range can glycine be used as an effective buffer due to its amino group?B) In a 0.1 M solution of glycine at pH 9.0, what fraction of glycine has its amino group in the -NH3+ form?C) How much 5 M KOH must be added to 1.0 L of 0.1 M glycine at pH 9.0 to bring its pH to exactly 10.0?D) When 99% of the glycine is in its -NH3+ form, what is the numerical relation between the pH of the solution and the pKa of the amino group?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, daniel1480
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced. be sure your answer has the correct number of significant digits.
Answers: 2


Chemistry, 22.06.2019 18:00, kingamir
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments....
Questions in other subjects:


Mathematics, 16.01.2020 15:31

Mathematics, 16.01.2020 15:31


Social Studies, 16.01.2020 15:31


Health, 16.01.2020 15:31


English, 16.01.2020 15:31

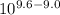
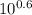
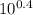
![pH = pKa + log [\dfrac{NH_2}{NH_3^+}]](/tpl/images/0687/6688/3d8e8.png)
![pH = pKa + log [\dfrac{0.01}{0.99}]](/tpl/images/0687/6688/7b228.png)


