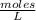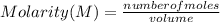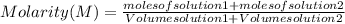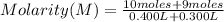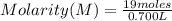Chemistry, 16.06.2020 23:57 joannamarquez0701
A student has two solutions of a substance. Solution-1: 25M, 400mL, and Solution-2: 30M, 300 ml. What is the molarity of the final solutions if these two solutions are mixed?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, NREYESLDS2806
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
A student has two solutions of a substance. Solution-1: 25M, 400mL, and Solution-2: 30M, 300 ml. Wha...
Questions in other subjects:

Health, 28.09.2019 09:20




Mathematics, 28.09.2019 09:20




Mathematics, 28.09.2019 09:20

Physics, 28.09.2019 09:30