
Chemistry, 16.06.2020 20:57 miagiancarlo
Describe what is happening in this chemical equation: Al2(SiO3)3 + NaOH → Na(SiO3)3 + Al2OH Please Help ASAP WILL MARK BRAINLIEST AND GIVE 5 STARS.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
Describe what is happening in this chemical equation: Al2(SiO3)3 + NaOH → Na(SiO3)3 + Al2OH Please H...
Questions in other subjects:


History, 20.09.2020 18:01

Biology, 20.09.2020 18:01


Chemistry, 20.09.2020 18:01



Mathematics, 20.09.2020 18:01


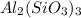 separates into
separates into 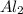 and
and 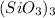 , while
, while 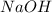 separates into
separates into 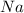 and
and 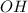 , and then they recombine with the other compound.
, and then they recombine with the other compound. 

