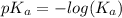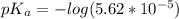Chemistry, 16.06.2020 19:57 Rachaeltice8810
Since some amino acids, such as Glu and His, can be viewed as weak acids or bases, the ratio of their protonated form to their deprotonated form is dependent on pH. Furthermore, you can use this knowledge to predict whether a given amino acid will exist predominantly in its protonated or deprotonated form at a given pH. To do this, it is easier to think about the pK, of the amino acid functional group. Recall from general chemistry that the pK, is equal to -log(K). Using the K, value for the Glu side chain, which is 5.62 x 10-5, calculate the pK, for the Glu side chain. pKa =

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2


Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Since some amino acids, such as Glu and His, can be viewed as weak acids or bases, the ratio of thei...
Questions in other subjects:

English, 04.08.2019 22:30




Biology, 04.08.2019 22:30

Mathematics, 04.08.2019 22:30



History, 04.08.2019 22:30


 value of Glu is
value of Glu is 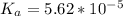
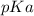 is mathematically represented as
is mathematically represented as