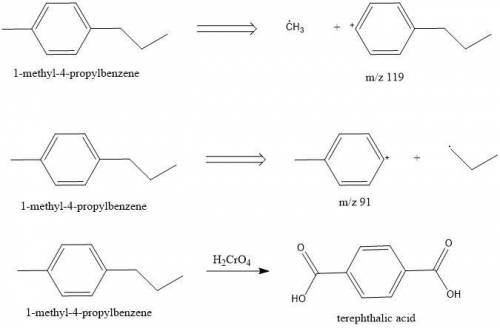
Compounds A and B (both C10H14) show prominent peaks in their mass spectrum at m/z 134 and 119. Compound B also shows a less prominent peak at m/z 91. On vigorous oxidation with chromic acid, compound A is nonreactive while compound B yielded terephthalic acid.
From this information, deduce the structures of both compounds, and then draw the structure of B.
You do not have to consider stereochemistry
You do not have to explicitly draw H atoms

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

You know the right answer?
Compounds A and B (both C10H14) show prominent peaks in their mass spectrum at m/z 134 and 119. Comp...
Questions in other subjects:




Chemistry, 25.01.2020 09:31


Mathematics, 25.01.2020 09:31

History, 25.01.2020 09:31


History, 25.01.2020 09:31