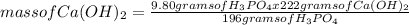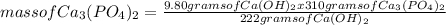Chemistry, 16.06.2020 07:57 perezsamantha3oqr0za
What is the maximum amount of Ca3(PO4)2 that can be prepared from 9.80 g of Ca(OH)2 and 9.80 g of
H3PO4
Ca(OH)2 (s) + H3PO4 (aq)
Ca3(PO4)2 (aq) + H2O (1)
balance the equation 1st.
O 6.80 g
O 15.5 g
O 8.60 g
o 13.7 g
O 10.3 g

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 08:00, ggdvj9gggsc
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
What is the maximum amount of Ca3(PO4)2 that can be prepared from 9.80 g of Ca(OH)2 and 9.80 g of
H...
Questions in other subjects:

History, 13.10.2019 23:30


English, 13.10.2019 23:30

Mathematics, 13.10.2019 23:30





History, 13.10.2019 23:30

Mathematics, 13.10.2019 23:30