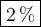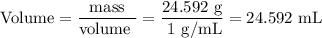Chemistry, 16.06.2020 01:57 elisakgarcia2007
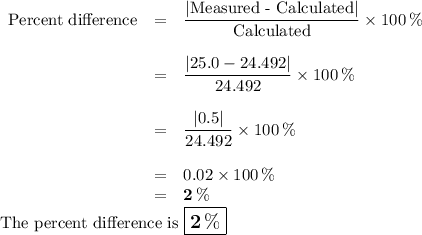
DATA AND CALCULATIONS: (you must show your calculations) Part I. Determination of accuracy of a graduated cylinder Calculations: Experimental Step Measurable Mass of empty graduated cylinder 47.229 g Mass of filled graduated cylinder 71.821 g Mass of water (filled – empty) g Volume of water, calculated (calculated from mass of water, using the equation “density = mass/volume”, given the fact that the density of water is exactly 1 g/mL) mL Volume of water, measured (from the reading of the scale on the graduated cylinder) 25.0 mL Percent difference between measured and calculated volumes of water [(measured-calculated)/calculated] ×100% %

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, dice50
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
DATA AND CALCULATIONS: (you must show your calculations) Part I. Determination of accuracy of a grad...
Questions in other subjects:






Mathematics, 19.05.2021 17:30


English, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30