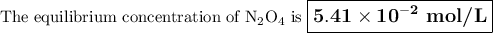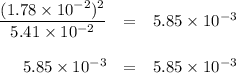Consider the following equilibrium:
N204(9)
2NO2(9)
Ket
= 5.85 x 10-3
Whi...

Chemistry, 16.06.2020 01:57 Aliciaonfleek
Consider the following equilibrium:
N204(9)
2NO2(9)
Ket
= 5.85 x 10-3
Which statement about this system is true?
Options:
The equilibrium lies to the left.
The equilibrium lies to the right.
If the equilibrium concentration of No, is 1.78 x 10-2 m, the equilibrium concentration of
N204 is
Options:
3.04 M.
1.85 × 10^-6 M.
5.42 × 10^-2 M.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 02:00, xbeatdroperzx
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 02.10.2020 15:01

English, 02.10.2020 15:01


Health, 02.10.2020 15:01



Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

English, 02.10.2020 15:01

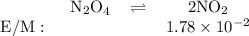
![K_{\text{c}} = \dfrac{\text{[NO$_{2}$]}^{2}}{\text{[N$_{2}$O$_{4}$]}} = \dfrac{(1.78 \times 10^{-2})^{2}}{\text{[N$_{2}$O$_{4}$]}} = 5.85 \times 10^{-3}\\\\\begin{array}{rcl}\\(1.78 \times 10^{-2})^{2}&=&\text{[N$_{2}$O$_{4}$]} \times 5.85 \times 10^{-3}\\\text{[N$_{2}$O$_4$]}&=& \dfrac{(1.78 \times 10^{-2})^{2}}{5.85 \times 10^{-3}}\\\\& = & \mathbf{5.41 \times 10^{-2}}\textbf{ mol/L}\\\end{array}\\](/tpl/images/0686/6303/751d3.png)