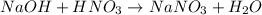Chemistry, 14.06.2020 09:57 brownzackery71
Which combination of reactants would result in a neutralization reaction with sodium nitrate,
NaNO3, as one of the products?
(A) Mg(NO3)2 + NaOH
(B) HNO, + NaOH
(C) CH4OH + NaOH
(D) HNO3 + NaCl

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Which combination of reactants would result in a neutralization reaction with sodium nitrate,
NaNO3...
Questions in other subjects:



Mathematics, 18.01.2021 14:00

Arts, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00