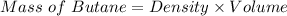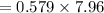Be sure to answer all parts. Butane gas is compressed and used as a liquid fuel in disposable cigarette lighters and lightweight camping stoves. Suppose a lighter contains 7.96 mL of butane (d = 0.579 g/mL). (a) How many grams of oxygen are needed to burn the butane completely?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
Be sure to answer all parts. Butane gas is compressed and used as a liquid fuel in disposable cigare...
Questions in other subjects:


Mathematics, 28.07.2021 18:50








Chemistry, 28.07.2021 19:00