
Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If the reaction starts with 2.15 mol of hydrogen bromide in 1.0 liter, and decomposes to 36.7%, what is the equilibrium constant of the decomposition of hydrogen bromide

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If...
Questions in other subjects:

Mathematics, 07.03.2021 19:00

Mathematics, 07.03.2021 19:00


World Languages, 07.03.2021 19:10


Advanced Placement (AP), 07.03.2021 19:10

Mathematics, 07.03.2021 19:10



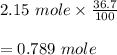
![K_c=\frac{[H_2][Br_2]}{[HBr]^2} \\\\=\frac{(0.395)(0.395)}{(1.361)^2} \\\\=\frac{0.156025}{1.852321} \\\\=0.084](/tpl/images/0685/5664/e5d29.png)



