
Chemistry, 13.06.2020 22:57 taylorruiz16
1.02 mg of a compound that is known to absorb at 340 nm is dissolved and diluted to 5.00 mL in a volumetric flask. A 1.00 mL aliquot (portion) of this solution was withdrawn from this flask, placed in a 10.00 mL volumetric flask and diluted to the mark. Some of this solution from the 10.00 mL flask was placed in a cuvet and analyzed analyzed spectroscopically. From these measurements it was found that the concentration was 6.97 x 10-5 M. Calculate the concentration of the analyte in the 5-mL flask

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, maevemboucher78
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 21:30, MJyoungboy
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
1.02 mg of a compound that is known to absorb at 340 nm is dissolved and diluted to 5.00 mL in a vol...
Questions in other subjects:

English, 21.01.2020 04:31


Mathematics, 21.01.2020 04:31

Mathematics, 21.01.2020 04:31

Mathematics, 21.01.2020 04:31

History, 21.01.2020 04:31

Mathematics, 21.01.2020 04:31

History, 21.01.2020 04:31

Mathematics, 21.01.2020 04:31

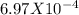 M
M


