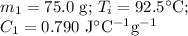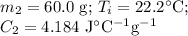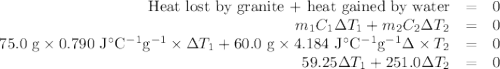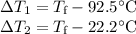Chemistry, 12.06.2020 21:57 dinosaur10
A 75.0 g sample of granite initially at 92.5oC is immersed into 60.0 g of water initially at 22.2oC. Determine the final temperature (in oC) when they reach thermal equilibrium. Assume no heat loss to the surroundings. Specific heats: granite = 0.790 J/(goC), water = 4.184 J/goC

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
A 75.0 g sample of granite initially at 92.5oC is immersed into 60.0 g of water initially at 22.2oC....
Questions in other subjects:





History, 05.05.2020 06:13

English, 05.05.2020 06:13

Mathematics, 05.05.2020 06:13


Mathematics, 05.05.2020 06:13

Mathematics, 05.05.2020 06:13