
Chemistry, 13.06.2020 09:57 hmskevinjacobo5471
I NEED HELP PLEASE, THANKS!
Ammonia (NH3) is an example of a Brønsted-Lowry Base.
-Define the Brønsted-Lowry acid-base theory.
-What is the pH of an ammonia solution that has a concentration of 0.335 M? The Kb of ammonia is 1.8 × 10^–5.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1


Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
I NEED HELP PLEASE, THANKS!
Ammonia (NH3) is an example of a Brønsted-Lowry Base.
-Define the...
-Define the...
Questions in other subjects:



Mathematics, 09.04.2020 06:51

English, 09.04.2020 06:51

Mathematics, 09.04.2020 06:51


Mathematics, 09.04.2020 06:51

Mathematics, 09.04.2020 06:51




![\rm K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.335 - x} = 1.8 \times 10^{-5}](/tpl/images/0685/0322/975fe.png)
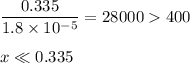
![\dfrac{x^{2}}{0.335} = 1.8 \times 10^{-5}\\\\x^{2} = 0.335 \times 1.8 \times 10^{-5}\\x^{2} = 6.03 \times 10^{-6}\\x = \sqrt{6.03 \times 10^{-6}}\\x = \text{[OH]}^{-} = \mathbf{2.46 \times 10^{-3}} \textbf{ mol/L}](/tpl/images/0685/0322/0aa42.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(2.46 \times 10^{-3}) = 2.61](/tpl/images/0685/0322/acbc3.png)



