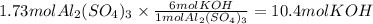Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 11:00, blondieb1722
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2
You know the right answer?
How many moles of potassium hydroxide are needed to completely react with 1.73 moles of aluminum sul...
Questions in other subjects:

Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01


French, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Biology, 18.10.2020 14:01


Geography, 18.10.2020 14:01