Chemistry, 12.06.2020 12:57 milkshakegrande101
. 125g of water has an initial temperature of 25.6°C, and is heated by 50.0g of a metal
which has been heated to 115.0°C. The metal heats the water so that both the metal
and the water reach a final temperature of 29.3°C. Calculate the specific heat of the
metal.


Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
. 125g of water has an initial temperature of 25.6°C, and is heated by 50.0g of a metal
which has b...
Questions in other subjects:


English, 15.10.2019 02:40



Mathematics, 15.10.2019 02:40

History, 15.10.2019 02:40


Mathematics, 15.10.2019 02:40


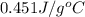
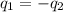
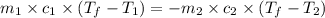
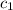 = specific heat of metal = ?
= specific heat of metal = ?
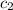 = specific heat of water =
= specific heat of water = 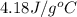
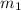 = mass of metal = 50.0 g
= mass of metal = 50.0 g
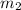 = mass of water = 125 g
= mass of water = 125 g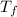 = final temperature of mixture =
= final temperature of mixture = 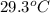
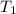 = initial temperature of metal =
= initial temperature of metal = 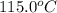
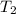 = initial temperature of water =
= initial temperature of water = 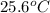
![(50.0g)\times c_1\times (29.3-115.0)^oC=-[(125g)\times 4.18J/g^oC\times (29.3-25.6)^oC]](/tpl/images/0684/0377/2c651.png)