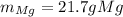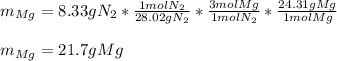Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, cicimarie2018
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

You know the right answer?
Magnesium and nitrogen react in a combination reaction to produce magnesium nitride:
3 Mg + N2 → Mg...
Questions in other subjects:


Mathematics, 07.05.2021 18:30


Mathematics, 07.05.2021 18:30


Mathematics, 07.05.2021 18:30



Biology, 07.05.2021 18:30