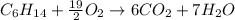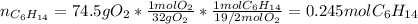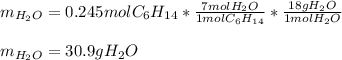Problem PageQuestion Liquid hexane CH3CH24CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. Suppose 60. g of hexane is mixed with 74.5 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Problem PageQuestion Liquid hexane CH3CH24CH3 will react with gaseous oxygen O2 to produce gaseous c...
Questions in other subjects:



Spanish, 28.01.2020 10:31


Mathematics, 28.01.2020 10:31


Computers and Technology, 28.01.2020 10:31

History, 28.01.2020 10:31

Mathematics, 28.01.2020 10:31