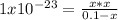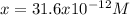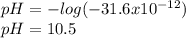Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 06:00, kelyanthecrafte
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
consider an exceptionally weak acid, HA, with Ka= 1 x 10-20. you make 0.1M solution of the salt NA....
Questions in other subjects:

Biology, 01.11.2019 17:31



Biology, 01.11.2019 17:31

Mathematics, 01.11.2019 17:31

Geography, 01.11.2019 17:31

Chemistry, 01.11.2019 17:31



History, 01.11.2019 17:31

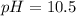
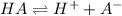
![Ka=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0682/3092/39962.png)
 due to the reaction extent is:
due to the reaction extent is: