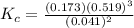Chemistry, 10.06.2020 18:57 ninaaforever
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a tank with of ammonia gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 13. mol. Calculate the concentration equilibrium constant for the decomposition of ammonia at the final temperature of the mixture.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:30, kaitie60
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions in other subjects:

Mathematics, 27.08.2019 21:30

English, 27.08.2019 21:30

Mathematics, 27.08.2019 21:30



Mathematics, 27.08.2019 21:30


Geography, 27.08.2019 21:30


Mathematics, 27.08.2019 21:30

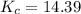
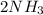 ↔
↔ 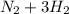
![[NH_3] = \frac{n_1}{V_1} = \frac{29}{75}](/tpl/images/0681/9995/b151c.png)
![[NH_3] = 0.387 \ M](/tpl/images/0681/9995/5ee5d.png)
![[N_2] = \frac{n_2}{V_2}](/tpl/images/0681/9995/a9b63.png)
![[N_2] = 0.173 \ M](/tpl/images/0681/9995/f89ee.png)
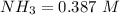

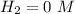
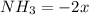 (this implies that it losses two moles of concentration )
(this implies that it losses two moles of concentration )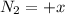 (this implies that it gains 1 moles)
(this implies that it gains 1 moles) (this implies that it gains 3 moles)
(this implies that it gains 3 moles)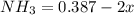
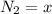
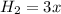
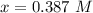
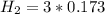

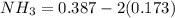
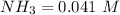
![K_c = \frac{[N_2][H_2]^3}{[NH_3]^2}](/tpl/images/0681/9995/10463.png)