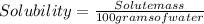A chemistry student is given of a clear aqueous solution at . He is told an unknown amount of a certain compound is dissolved in the solution. The student allows the solution to cool to . At that point, the student sees that a precipitate has formed. He pours off the remaining liquid solution, throws away the precipitates, and evaporates the water from the remaining liquid solution under vacuum. More precipitate forms. The student washes, dries and weighs the additional precipitate. It weighs 50,0 g. Using only the information above, can you calculate the solubility of X in water at 16. C. If you said yes, calculate it.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

You know the right answer?
A chemistry student is given of a clear aqueous solution at . He is told an unknown amount of a cert...
Questions in other subjects:






Computers and Technology, 27.11.2019 21:31