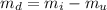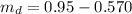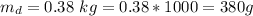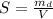Chemistry, 10.06.2020 06:57 cguzman4993
A chemistry student weighs out of an unknown solid compound and adds it to of distilled water at . After minutes of stirring, only some of the has dissolved. The student drains off the solution, then washes, dries and weighs the that did not dissolve. It weighs 0.570 kg.
Required:
a. Using the information above, can you calculate the solubility of X?
b. If so, calculate it. Remember to use the correct significant digits and units. .

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
A chemistry student weighs out of an unknown solid compound and adds it to of distilled water at . A...
Questions in other subjects:



Social Studies, 12.01.2021 03:00


Business, 12.01.2021 03:00

Arts, 12.01.2021 03:00



Mathematics, 12.01.2021 03:00

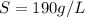
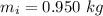
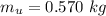
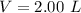
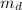 ) is mathematically represented as
) is mathematically represented as