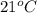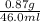Chemistry, 09.06.2020 14:57 brennae8529
A geochemist in the field takes a 46.0 mL sample of water from a rock pool lined with crystals of a certain mineral compound X. He notes the temperature of the pool, 21°C, and caps the sample carefully. Back in the lab, the geochemist filters the sample and then evaporates all the water under vacuum. Crystals of X are left behind. The researcher washes, dries and weighs the crystals. They weigh 0.87 g.
Required:
Using only the information above, can you calculate the solubility of X in water at 21°C? If yes, calculate it.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
A geochemist in the field takes a 46.0 mL sample of water from a rock pool lined with crystals of a...
Questions in other subjects:


Mathematics, 03.02.2021 21:50


Advanced Placement (AP), 03.02.2021 21:50

Mathematics, 03.02.2021 21:50

Mathematics, 03.02.2021 21:50


Chemistry, 03.02.2021 21:50

Social Studies, 03.02.2021 21:50

 g/ml.
g/ml.