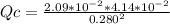Consider the following reaction where Kc = 1.80×10-2 at 698 K:
2HI(g) → H2(g) + I2(g)
A reaction mixture was found to contain 0.280 moles of HI (g), 2.09×10^-2 moles of H2 (g), and 4.14×10^-2 moles of I2 (g), in a 1.00 liter container.
Required:
a. Is the reaction at equilibrium?
b. What direction must it run in order to reach equilibrium?
c. The reaction
1. must run in the forward direction to reach equilibrium.
2. must run in the reverse direction to reach equilibrium.
3. is at equilibrium.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, morrisjillian23
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2


Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
Consider the following reaction where Kc = 1.80×10-2 at 698 K:
2HI(g) → H2(g) + I2(g)
...
...
Questions in other subjects:


English, 26.08.2020 08:01

Mathematics, 26.08.2020 08:01


Mathematics, 26.08.2020 08:01

Mathematics, 26.08.2020 08:01

Mathematics, 26.08.2020 08:01


Mathematics, 26.08.2020 08:01

![Qc=\frac{[C]^{c}*[D]^{d} } {[A]^{a}*[B]^{b}}](/tpl/images/0680/9750/5af8a.png)
![Qc=\frac{[H_{2} ]*[I_{2} ] } {[HI]^{2}}](/tpl/images/0680/9750/8091f.png)
![[H_{2} ]=\frac{2.09*10^{-2} moles}{1 Liter}](/tpl/images/0680/9750/734fa.png) =2.09*10⁻²
=2.09*10⁻² 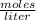
![[I_{2} ]=\frac{4.14*10^{-2} moles}{1 Liter}](/tpl/images/0680/9750/92c8b.png) =4.14*10⁻²
=4.14*10⁻² ![[I_{2} ]=\frac{0.280 moles}{1 Liter}](/tpl/images/0680/9750/e5237.png) = 0.280
= 0.280