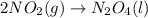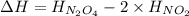Chemistry, 07.06.2020 13:57 Spence8900
PLZ HELP For the reaction: 2NO2(g) → N2O4(l),
the ΔH of the reactants, two moles of NO2 (g), is + 66 kJ/mol,
and the ΔH of the products, N2O4 (l), is -20 kJ/mol.
Which of the following shows the ΔH (change in enthalpy) for the reaction as a whole?
Question 8 options:
ΔrxnH =(- 20 kJ/mol) / (+66 kJ/mol)
ΔrxnH = (+66 kJ/mol) + (- 20 kJ/mol)
ΔrxnH =(-20 kJ/mol) - (+ 66 kJ/mol)
ΔrxnH =(+66 kJ/mol) - (- 20 kJ/mol)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2


Chemistry, 22.06.2019 23:30, adamgala3885
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
PLZ HELP For the reaction: 2NO2(g) → N2O4(l),
the ΔH of the reactants, two moles of NO2 (g), is + 6...
Questions in other subjects:

Mathematics, 16.12.2020 04:40


Social Studies, 16.12.2020 04:40

Chemistry, 16.12.2020 04:40

Mathematics, 16.12.2020 04:40


Health, 16.12.2020 04:40


Social Studies, 16.12.2020 04:40

Mathematics, 16.12.2020 04:40

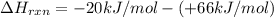
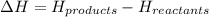
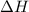 = enthalpy change = ?
= enthalpy change = ?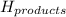 = enthalpy of products
= enthalpy of products 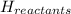 = enthalpy of reactants
= enthalpy of reactants