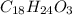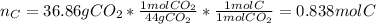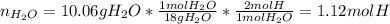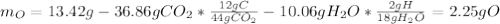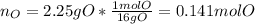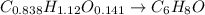Chemistry, 07.06.2020 05:00 calebwoodall6477
Combustion analysis of a 13.42-g sample of an unknown organic compound (which contains only carbon, hydrogen, and oxygen) produced 36.86 g CO2 and 10.06 g H2O. The molar mass of the compound is 288.38 g/mol . Part A Find the molecular formula of the unknown compound. Express your answer as a chemical formula.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3


You know the right answer?
Combustion analysis of a 13.42-g sample of an unknown organic compound (which contains only carbon,...
Questions in other subjects:

Mathematics, 23.11.2019 10:31


Mathematics, 23.11.2019 10:31

Mathematics, 23.11.2019 10:31

English, 23.11.2019 10:31


Social Studies, 23.11.2019 10:31

Mathematics, 23.11.2019 10:31

History, 23.11.2019 10:31