Chemistry, 06.06.2020 05:01 kestegag7162
2KMnO4= K2MnO4+ MnO2+O2 how many grams of KMnO4 are required to produce 1.60 grams of O2

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, sannai0415
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3


Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
You know the right answer?
2KMnO4= K2MnO4+ MnO2+O2 how many grams of KMnO4 are required to produce 1.60 grams of O2...
Questions in other subjects:

Mathematics, 27.01.2021 02:50


Mathematics, 27.01.2021 02:50


Mathematics, 27.01.2021 02:50

English, 27.01.2021 02:50



Arts, 27.01.2021 02:50

 will be required to produce 1.60 grams of
will be required to produce 1.60 grams of 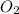



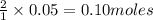 of
of