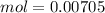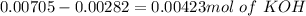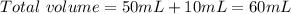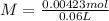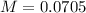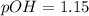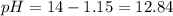Chemistry, 06.06.2020 03:59 genyjoannerubiera
A 50.0 mL solution of 0.141 M KOH is titrated with 0.282 M HCl . Calculate the pH of the solution after the addition of each of the given amounts of HCl .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 16:50, mathiscool7
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 14:00, LeoInc6806
[07.06] which of the following chemical reactions is an oxidation-reduction reaction? (2 points) wo3 + 3h2 yields w + 3h2o kno3 + licl yields lino3 + kcl caso4 + 2nacl yields na2so4 + cacl2 mg(no3)2 + 2hbr yields mgbr2 + 2hno3
Answers: 1
You know the right answer?
A 50.0 mL solution of 0.141 M KOH is titrated with 0.282 M HCl . Calculate the pH of the solution af...
Questions in other subjects:




Mathematics, 31.08.2019 02:50

English, 31.08.2019 02:50



Biology, 31.08.2019 02:50

Mathematics, 31.08.2019 02:50

Social Studies, 31.08.2019 02:50



 and we have the concentration of the HCl
and we have the concentration of the HCl 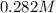 , when we plug the values into the equation we got:
, when we plug the values into the equation we got:
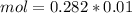

 and
and  ).
).