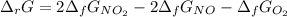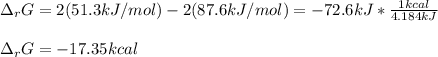(A) - 9.3 kcal

Chemistry, 06.06.2020 02:57 glowbaby123
Calculate the free energy change for the reaction:
2 NO(g) + O2(g) → 2 NO2(g)
(A) - 9.3 kcal
(B) + 24.9 kcal
(C) + 9.3 kcal
(D) - 16.6 kcal
(E) + 16.6 kcal


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1


Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Calculate the free energy change for the reaction:
2 NO(g) + O2(g) → 2 NO2(g)
(A) - 9.3 kcal
(A) - 9.3 kcal
Questions in other subjects:


Engineering, 30.04.2021 14:30

Biology, 30.04.2021 14:30




English, 30.04.2021 14:30