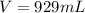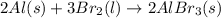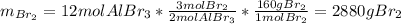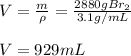Chemistry, 05.06.2020 08:58 codycollier
Consider the following unbalanced equation. How many Liters of bromine are needed to produce 12 moles of Aluminum bromide? The density of bromine is 3.1 g/mL. Al (s) + Br2 (l)= AlBr3 (s)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 10:30, mv603177
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

You know the right answer?
Consider the following unbalanced equation. How many Liters of bromine are needed to produce 12 mole...
Questions in other subjects:

Chemistry, 19.07.2019 16:50

History, 19.07.2019 16:50

Chemistry, 19.07.2019 16:50

Mathematics, 19.07.2019 16:50

Biology, 19.07.2019 16:50

History, 19.07.2019 16:50



Biology, 19.07.2019 16:50