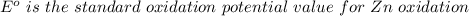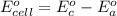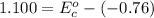Chemistry, 04.06.2020 13:20 saggin2454
Using the cell voltage measured for the first cell studied, with cell chemistry Zn/Zn^2+ \ Cu^2+/Cu, and the known half life potential for Zn^2/Zn calculate the reduction potential for Cu^2+/Cu and enter value below.
The information received for this problem were the values obtained during an online lab:
Cu xM cell voltage:1.100 V
Range: 0.005 V
Temp: 25 degrees C

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 23.06.2019 08:30, maddycat4005
Which can be observed only in a microscopic view? a) structure of a muscle cell b) shape of a soybean plant c) foam insulation d) x-ray of a knee joint
Answers: 2
You know the right answer?
Using the cell voltage measured for the first cell studied, with cell chemistry Zn/Zn^2+ \ Cu^2+/Cu,...
Questions in other subjects:

Social Studies, 02.12.2021 15:00

Mathematics, 02.12.2021 15:00



Mathematics, 02.12.2021 15:00

English, 02.12.2021 15:00

Chemistry, 02.12.2021 15:00



Physics, 02.12.2021 15:00

 is
is