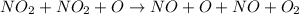The decomposition of nitrogen dioxide is described by the following chemical equation:
2NO_2...

The decomposition of nitrogen dioxide is described by the following chemical equation:
2NO_2 (g) > 2NO (g) + O_2 (g)
Suppose a two-step mechanism is proposed for this reaction, beginning with this elementary reaction:
NO_2 (g) > NO_(g) + O_(g)
Suppose also that the second step of the mechanism should be bimolecular.
a) Suggest a reasonable second step. That is, write the balanced chemical equation of a bimolecular elementary reaction that would complete the proposed mechanism.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

You know the right answer?
Questions in other subjects:


Chemistry, 08.09.2020 09:01




Mathematics, 08.09.2020 09:01

English, 08.09.2020 09:01

Mathematics, 08.09.2020 09:01


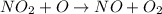 ".
".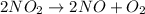
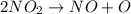 ...(equation 1)
...(equation 1)