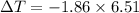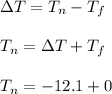What is the freezing point of a solution of 210.0 g of glycerol, formula C3H8O3, dissolved in 350. g of water? Careful. First get molar mass and use molar mass to determine molality concentration. Then use freeze pt. depression formula to determine the change in freezing pt. Then determine the new freeze point. The freezing point depression constant for water is Kf= -1.86 oCelcius/molal. Report your answer rounded to 1 decimal point and do not include units.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, Thomas7785
Two things that biomedical has invented or innovated
Answers: 1

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 01:30, Michael845313
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
What is the freezing point of a solution of 210.0 g of glycerol, formula C3H8O3, dissolved in 350. g...
Questions in other subjects:


History, 02.09.2019 16:30








Social Studies, 02.09.2019 16:40

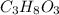 Freezing point depression constant for water, Kf = 0.512 °C/m
Freezing point depression constant for water, Kf = 0.512 °C/m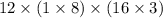
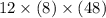
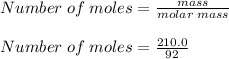
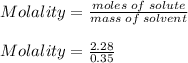
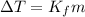
 is the change in temperature.
Kf is the molal freezing point constant.
m is the molality of solution.
is the change in temperature.
Kf is the molal freezing point constant.
m is the molality of solution.