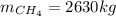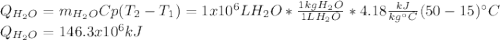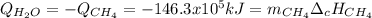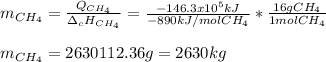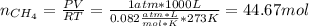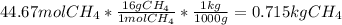En un balneario necesitan calentarse 1 millón de litros de agua anuales, subiendo la temperatura desde 15 ºC a 50 ºC y para ello utilizan calderas de gas natural (CH4), siendo la entalpía de combustión del metano de – 890 KJ/mol. Calcula:
a) El consumo anual de gas natural. Sol: 2630 Kg
b) El coste económico, anual, si el precio del m3, en condiciones normales, de gas natural es de 0,45 €. Datos Ce (H2O) = 4,18 KJ/Kg·K
Sol: 1657 €.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
En un balneario necesitan calentarse 1 millón de litros de agua anuales, subiendo la temperatura des...
Questions in other subjects:



Mathematics, 26.02.2021 16:40




Mathematics, 26.02.2021 16:40

World Languages, 26.02.2021 16:40


English, 26.02.2021 16:40