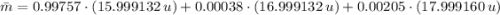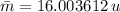Chemistry, 02.06.2020 04:58 GreenHerbz206
El oxigeno natural se compone de un 99.757% de 160 con una masa de 15.999132u . 0.038% de 170 con una masa de 16.999132u y 0.205% de 180 que posee una masa de 17.999160u. Calcule la masa atomica promedio del oxigeno (AO).

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 02:00, Turtlelover05
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
El oxigeno natural se compone de un 99.757% de 160 con una masa de 15.999132u . 0.038% de 170 con un...
Questions in other subjects:

Mathematics, 02.09.2019 22:20

English, 02.09.2019 22:20

Mathematics, 02.09.2019 22:20




Mathematics, 02.09.2019 22:30