
Chemistry, 01.06.2020 17:59 lilchannelll4125
Experiments have shown that the equilibrium constant Kp for the reaction NO(g) + NO2(g) ⇌ N2O3(g) is 0.03. A chamber with a constant total pressure initially contains NO with a partial pressure of 200 MPa, NO2 with a partial pressure of 250 MPa, and N2O3 with a partial pressure of 50 MPa. What will occur over time?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:40, Calumworthy6046
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Experiments have shown that the equilibrium constant Kp for the reaction NO(g) + NO2(g) ⇌ N2O3(g) is...
Questions in other subjects:

Mathematics, 05.02.2021 16:30

History, 05.02.2021 16:30

Mathematics, 05.02.2021 16:30


Biology, 05.02.2021 16:30

Mathematics, 05.02.2021 16:30




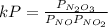 = 0.03
= 0.03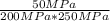 = 0.001
= 0.001

