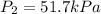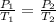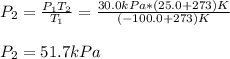Chemistry, 30.05.2020 19:02 hectorav6619
The temperature of a sample of gas in a steel
tank at 30.0 kPa is increased from -100.0°C to
25.0 °C. What is the final pressure inside the
tank?
A. 5.17 kPa
B. 51.7 kPa
C. 517 kPa
D. 5170 kPa

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
The temperature of a sample of gas in a steel
tank at 30.0 kPa is increased from -100.0°...
tank at 30.0 kPa is increased from -100.0°...
Questions in other subjects:





Mathematics, 30.04.2021 01:20

English, 30.04.2021 01:20

Mathematics, 30.04.2021 01:20

Mathematics, 30.04.2021 01:20