

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2

Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Hydrochloric acid reacts with lead (II) oxide to make lead (II) chloride and water. How many grams o...
Questions in other subjects:







Computers and Technology, 14.11.2019 17:31

Computers and Technology, 14.11.2019 17:31


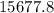 grams of HCl is required to produce 215 moles of lead (II) chloride
grams of HCl is required to produce 215 moles of lead (II) chloride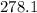 g/mol
g/mol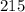 moles of lead (II) chloride is
moles of lead (II) chloride is 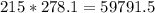
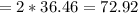 grams
grams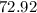 grams of HCL
grams of HCL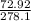 grams of HCL
grams of HCL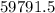 gram of PbO2 is formed by
gram of PbO2 is formed by 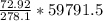 grams of HCL
grams of HCL

