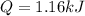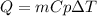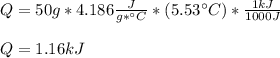Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
How much heat, in kJ, is required to raise the temperature of 50 g of water by 5.53°C? (Round to the...
Questions in other subjects:


Mathematics, 02.09.2020 23:01


Mathematics, 02.09.2020 23:01




Social Studies, 02.09.2020 23:01

English, 02.09.2020 23:01

Mathematics, 02.09.2020 23:01