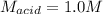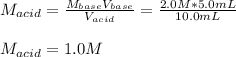Chemistry, 29.05.2020 20:01 nenelacayo07
In a titration, 5.0 ml of a 2.0 M NaOH (aq) solutions exactly neutralizes 10.0 ml of an HCL (aq) solution. what is the concentration of the HCl (aq) solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ElizabethF
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 09:00, valeriekbueno
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

You know the right answer?
In a titration, 5.0 ml of a 2.0 M NaOH (aq) solutions exactly neutralizes 10.0 ml of an HCL (aq) sol...
Questions in other subjects:


Physics, 18.12.2020 05:30

Mathematics, 18.12.2020 05:30

Mathematics, 18.12.2020 05:30

Mathematics, 18.12.2020 05:30

English, 18.12.2020 05:30