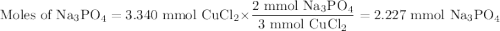Chemistry, 29.05.2020 09:57 hiddenauthors436
URGENT CHEMISTRY EXPERT!
Consider the reaction :2Na3PO4(aq) + 3CuCl2(aq) → Cu3(PO4)2(s) + 6NaCl(aq)
PART 1: What volume (in mL) of 0.300 MNa3PO4 solutions is needed to completely react with 16.7mLof 0.200M CuCl2?
PART 2: Find the net ionic equation for this reaction.

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 11:00, alejachico2
Suppose you increase your walking speed from 7 m/s to 15 m/s in a period of 1 s. what is your acceleration?
Answers: 1
You know the right answer?
URGENT CHEMISTRY EXPERT!
Consider the reaction :2Na3PO4(aq) + 3CuCl2(aq) → Cu3(PO4)2(s) + 6Na...
Consider the reaction :2Na3PO4(aq) + 3CuCl2(aq) → Cu3(PO4)2(s) + 6Na...
Questions in other subjects:

English, 25.09.2019 17:10

English, 25.09.2019 17:20

English, 25.09.2019 17:20


English, 25.09.2019 17:20

English, 25.09.2019 17:20

English, 25.09.2019 17:20



English, 25.09.2019 17:20