
Chemistry, 29.05.2020 05:00 Coltong121
One millimole of Ni(NO3)2 dissolves in 240.0 mL of a solution that is 0.500 M in ammonia. The formation constant of Ni(NH3)62+ is 5.5×108. What is the initial concentration of Ni(NO3)2 in the solution? What is the equilibrium concentration of Ni2+(aq ) in the solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:50, mi364
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2


Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
One millimole of Ni(NO3)2 dissolves in 240.0 mL of a solution that is 0.500 M in ammonia. The format...
Questions in other subjects:

Biology, 05.09.2019 22:20


English, 05.09.2019 22:20

Physics, 05.09.2019 22:20




Chemistry, 05.09.2019 22:20


Chemistry, 05.09.2019 22:20

![[Ni^{2+}]_0=0.0042M](/tpl/images/0669/8824/46776.png)
![[Ni^{2+}]_{eq}=0M](/tpl/images/0669/8824/028c2.png)
![Ni^{+2}+6NH_3\rightleftharpoons [Ni(NH_3)_6]^{2+}](/tpl/images/0669/8824/85f00.png)
![Kf=\frac{[Ni(NH_3)_6]^{2+}}{[NH_3]^6[Ni^{+2}]}](/tpl/images/0669/8824/f8603.png)
![[Ni^{2+}]_0=\frac{0.001mol}{0.240L} =0.0042M](/tpl/images/0669/8824/ca35e.png)
 due to the reaction extent:
due to the reaction extent: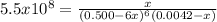
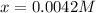
![[Ni^{2+}]_{eq}=0.0042M-x=0.0042M-0.0042M=0](/tpl/images/0669/8824/b9bd2.png)


